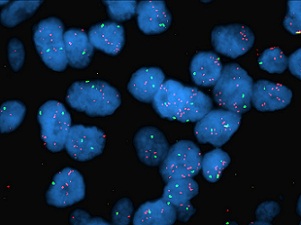
HER2 gene amplification in breast cancer
|
| |
| Contacts: |
Sharon Forrest Immuncytochemistry & Molecular Pathology Services Manager Tel: 0151 706 4485 Email: sharon.forrest@LiverpoolFT.nhs.uk Steven Forrest Molecular Pathology Team Leader Tel: 0151 706 4485 Email: steven.forrest@LiverpoolFT.nhs.uk Professor Sarah Coupland Consultant Histopathologist Email: s.e.coupland@liverpool.ac.uk Dr Helen Kalirai Liverpool Ocular Oncology Molecular Pathology Service Email: h.kalarai@liverpool.ac.uk Sophie Thornton Liverpool Ocular Oncology Molecular Pathology Service Email: sophie.thornton@LiverpoolFT.nhs.uk |
 HER2 gene amplification in breast cancer |