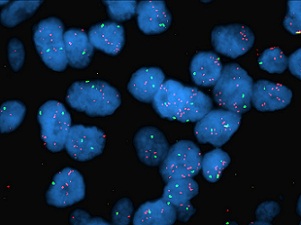
HER2 gene amplification in breast cancer
|
| |
| Contacts: |
Sharon Forrest Immunocytochemistry & Molecular Pathology Services Manager Tel: 0151 706 4485 Email: sharon.forrest@LiverpoolFT.nhs.uk Steven Forrest Molecular Pathology Scientific Section Lead Tel: 0151 706 4485 Email: steven.forrest@LiverpoolFT.nhs.uk Elisabete Martins Molecular Pathology Team Leader/CVLP Lead Tel: 0151 706 4485 Email: Elisabete.Martins@liverpoolft.nhs.uk Carolina Felix Molecular Pathology Team Leader Tel: 0151 706 4485 Email: Carolina.Felix@liverpoolft.nhs.uk For enquiries about results contact: LCL Customer Care Tel: 0151 706 5888 Email: LCLCustomerCare@LiverpoolFT.nhs.uk |
 HER2 gene amplification in breast cancer |